Testing of biocompatible 3D printed resins
Testing of biocompatible 3D printed resins is a critical step in assessing the suitability of printed parts for biomedical, dental and other application-critical uses. In photopolymer additive manufacturing, test results are not intrinsic properties of the liquid resin but outcomes of a complete material–process–testing system.
Correct interpretation of testing data requires understanding the influence of formulation, printing specifications, post-processing workflow and applied testing standards.
Purpose of biocompatibility testing
Biocompatibility testing is performed to evaluate the interaction between printed parts and biological systems under defined conditions. These tests are designed to assess potential biological responses and to support application-specific risk evaluation.
Test results are valid only for the specific material version and workflow under which they were generated and must be interpreted within their intended application scope.
Role of printing and processing conditions
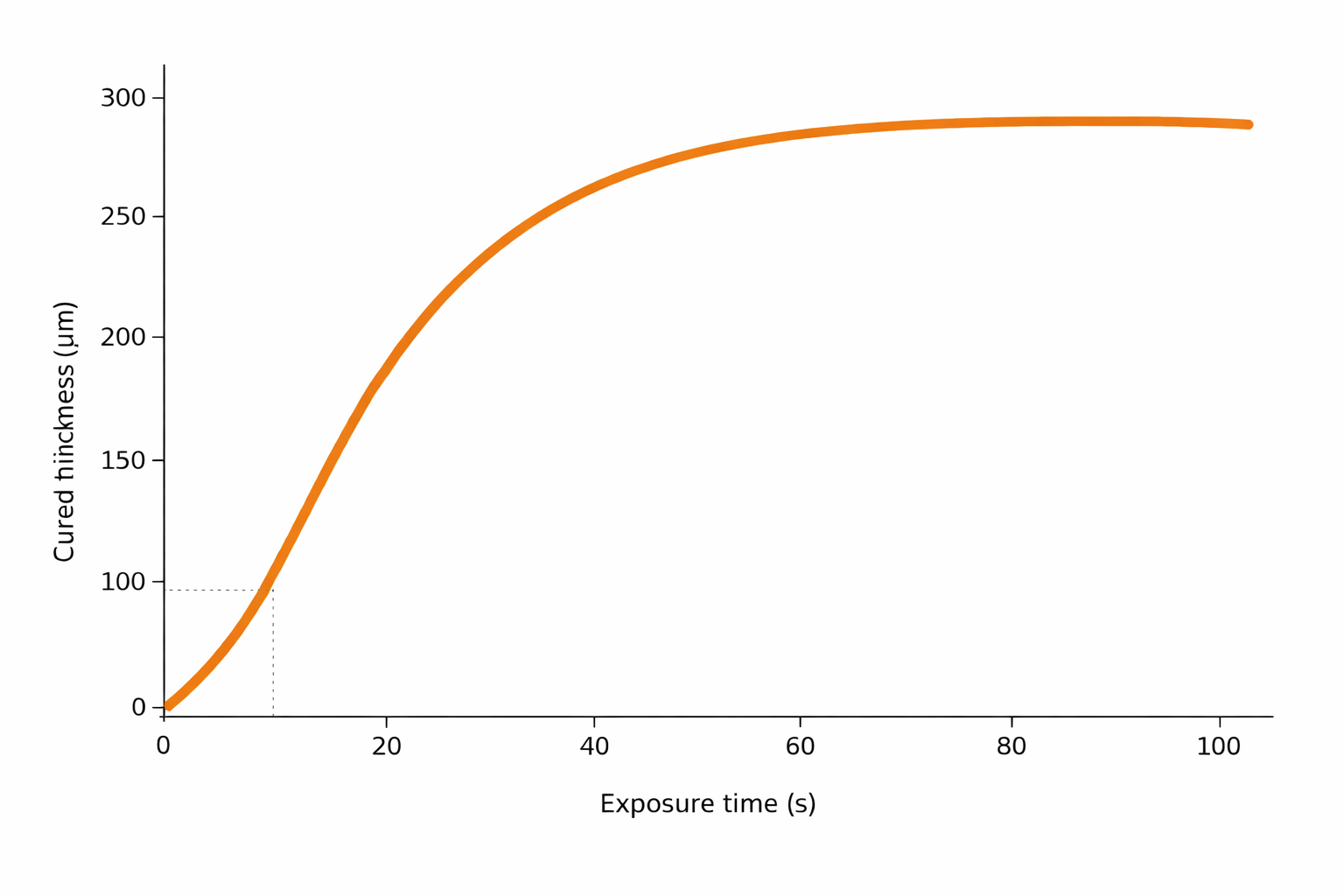
Printing specifications and post-processing workflows have a direct impact on the biological performance of printed parts. Parameters such as exposure strategy, orientation, washing efficiency and post-curing conditions influence polymer conversion, surface chemistry and residual extractables.
As a result, changes in printing or post-processing conditions can lead to significant variations in biocompatibility outcomes, even when the same resin formulation is used.
Influence of printer technology
Printer technology, including light source characteristics, wavelength distribution, optical uniformity and calibration state, affects energy delivery and polymer network formation.
Differences between printer architectures may therefore influence both mechanical behavior and biocompatibility results, reinforcing the need for printer-specific reference configurations during testing.
Testing standards and methodologies
Biocompatibility testing is performed according to recognized standards and methodologies that define specimen preparation, conditioning, exposure conditions and evaluation criteria.
While testing standards provide structured frameworks, they do not eliminate process dependency. Results obtained using different printers, workflows or specimen geometries should not be directly compared without considering these variables.
Interpretation and limitations of test results
Biocompatibility test results represent typical outcomes obtained under defined reference configurations. They do not constitute universal or intrinsic material properties independent of processing conditions.
Testing outcomes should be interpreted as part of a broader system-level evaluation that includes material selection, workflow control and application-specific requirements.
Relationship to validation and regulatory use
Biocompatibility testing supports validation activities but does not replace application-specific validation or regulatory assessment.
Responsibility for ensuring compliance with applicable regulations and standards rests with the device manufacturer or user implementing the material and workflow.
System-based approach to testing
3Dresyns® approaches biocompatibility testing within a system-based framework that recognizes the multivariable nature of photopolymer additive manufacturing.
Testing data are generated using qualified workflows and reference configurations to provide meaningful, reproducible and context-aware performance information.
Governing principle
Biocompatibility testing of 3D printed photopolymers reflects system-dependent outcomes. Test results represent typical responses obtained under defined printing and post-processing configurations, not intrinsic properties of the liquid resin alone.